Computational Synthesis Paving the Way for Materials by Design Reality

Dr. Wenhao Sun stands as a testament to tenacity and ingenuity in the realm of computational materials science. A Dow Early Career Professor of Materials Science and Engineering at the University of Michigan, Sun got his start pursuing dual degrees in applied math and material science at Northwestern University before moving onto the Massachusetts Institute of Technology for his PhD. He joined Lawrence Berkeley National Laboratory as a postdoc and eventually staff scientist.
Sun has always been intrigued by the challenge of synthesizing materials. His research group at U-M aims to resolve the fundamental scientific problems hindering the computational materials design process.

“I’ve always been in the field of computational materials, discovery and design, and the idea is that we should find new materials or find new applications for known materials.”
The group uses quantum mechanical calculations, applied thermodynamics, and machine learning models to deepen our fundamental understanding of the way materials are made, how their atoms are arranged in space, and how they behave in various conditions, all while exploring new chemical spaces for functional materials that could lead to technological developments.
“I’ve always been in the field of computational materials, discovery and design, and the idea is that we should find new materials or find new applications for known materials,” said Sun.
“When I started my PhD, there was a program being announced that was the Materials Genome Initiative, which is this idea that we can use computation to accelerate material discovery and design.”
It was this initiative that spurred Sun’s extensive involvement in computational materials science. Beyond the prediction of new materials, Sun was driven by the deeper question of how to synthesize materials that only existed theoretically—those that had been computationally predicted to have superior properties, but for which no practical synthesis methods were yet known. This fascination recently brought Sun and researchers in his lab to the doorstep of a 200-year-old question that has given pause to generations of geologists and stumped materials scientists: How does dolomite form?
Dolomite is a mineral composed primarily of calcium magnesium carbonate (CaMg(CO₃)₂). It forms a part of the carbonate mineral group and is commonly found in sedimentary rock sequences. Dolomite beds were initially formed when ancient seas covered large swaths of what are now continents. Over millions of years, layers of calcium and magnesium carbonates precipitated from the seawater, forming sedimentary deposits.
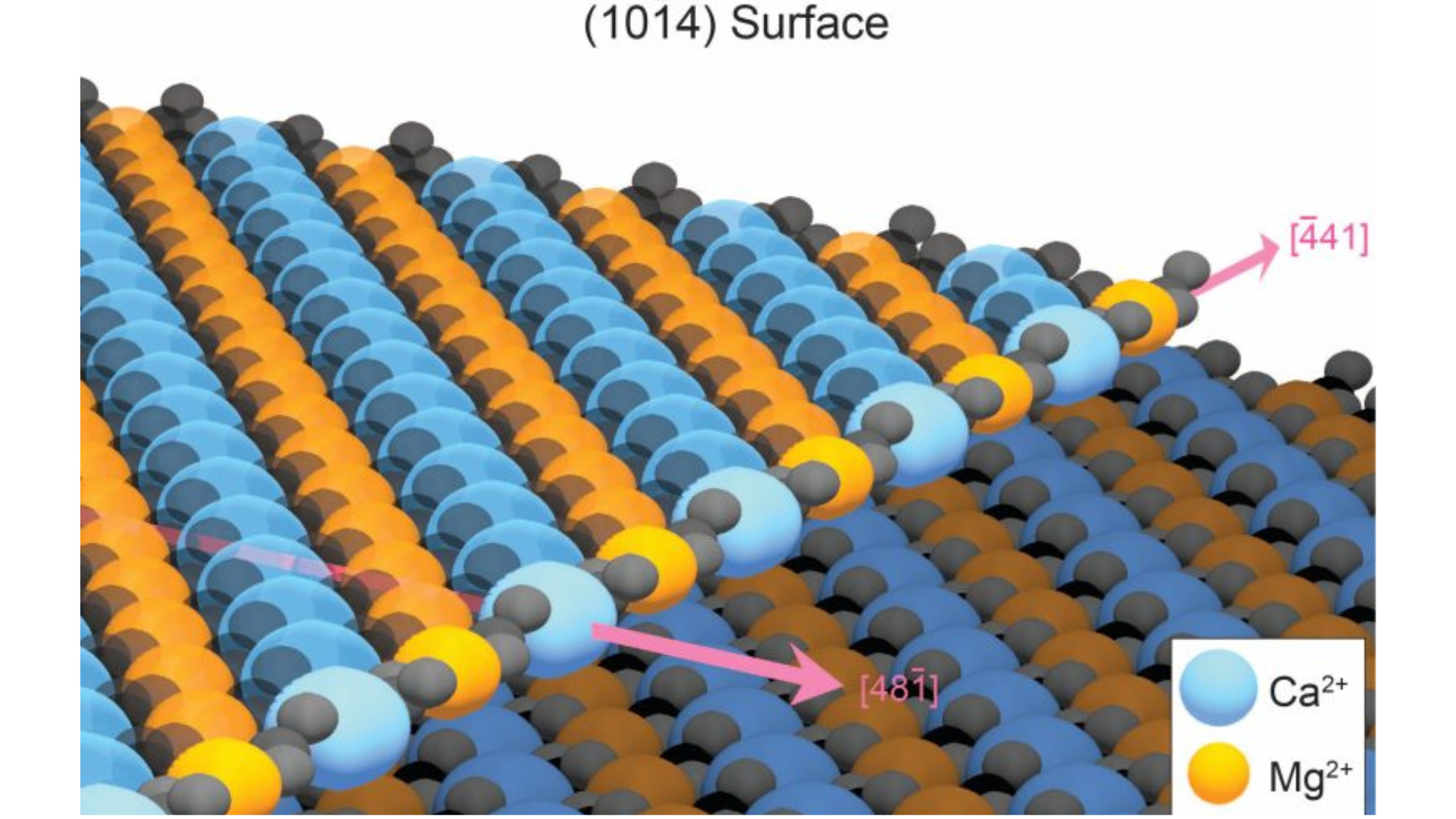
“It’s actually funny, diving into the dolomite problem because it’s a problem that most people will have never heard of, my [younger] self included. It’s like magic. It’s a mystery. We don’t know how it forms. There’s mountains of it, but we don’t know where it came from,” said Sun.
“That’s why computation was so important in that project. We can do things here, we can test hypotheses and examine numbers that experimentally would be impossible to get otherwise.”
The Sun Research Group used simulations to study dolomite formation. These simulations took advantage of sophisticated software developed at U-M’s Predictive Structure Materials Science (PRISMS) Center, enabling quick and efficient analysis. Their research identified a major barrier to the formation of dolomite: defects in the crystal structure during normal growth. These defects happen when calcium and magnesium ions attach randomly to the growing dolomite crystal. Often, these ions end up in incorrect positions, which hinders further crystal growth.
The key insight was understanding that these defects were not permanent. Simulations showed that these disordered atoms could be the first to dissolve when the crystal was washed with water. By applying a process of repeated rinsing, simulating natural precipitation and erosion, the defects could be removed, allowing for proper layer formation.
This breakthrough extends beyond pure geology. It presents potential benefits for the fields of engineering and technology. By demonstrating that defects can be periodically dissolved throughout the materials synthesis process, Sun’s work could enhance the speed and quality of technological materials for advanced semiconductors, solar panels, and batteries. Currently, Sun’s insights are being applied to various consulting projects focused on manufacturing large semiconductor wafers using dissolution and growth cycles.
“What I want to do is take these minerals that have inspired us from nature, learn the key underlying physical principles, and use them to grow technological materials that we care about today,” Sun says.
Sun describes his main craft as research, but expresses the importance of seeking inspiration from elsewhere. He mentions concerning himself less and less with publishing a high volume of papers and findings, but instead in seeking meaningful breakthroughs and pursuing projects that naturally spark his and his team’s curiosity.
Outside of his breakthrough contributions to materials science, Sun finds a tremendous amount of inspiration in the arts. This passion developed when he and his wife developed a habit of visiting local art museums during their travels to scientific conferences during his graduate studies and postdoc career.
Sun draws a distinct parallel between the meticulous work of artists like Da Vinci and his approach to research. “How many paintings do you think Da Vinci made in his lifetime? Fifteen,” he points out, emphasizing Da Vinci’s diverse expertise and attention to perfection.
Sun strives to capture that same perfection in his work. “I started coming around to the conclusion that I should try to make Da Vinci-like paintings. Everyone says, ‘Get your paper out. Perfection is the opposite of good.’ But to Da Vinci, good was the enemy of perfection,” says Sun.
Sun promises an exciting period of “show-stopping” papers and contributions from his team. On the heels of the Dolomite breakthrough, the Sun Research Group has several other long-term projects wrapping up, including a study on Quasicrystals — puzzlingly unique forms of matter that have order in their atomic patterns, but do not repeat that order in their crystal structure.
Regardless of what Sun and his team publish next, one thing is sure: his dedication to pushing the boundaries of materials science, and approach to conducting thoughtful and thorough research with the same care as a master craftsman are sure to lead to results and findings that inspire, intrigue, and stand the test of time.